
Thermochemical processes
One type of thermal process uses the energy stored in such resources as coal or biomass to simply release the hydrogen contained within their molecular structures.
Another type uses heat in combination with closed chemical cycles to produce hydrogen from feedstocks, such as water; these are known as “thermochemical” processes.
• Distributed Natural Gas Reforming
• Bio-Derived Liquids Reforming
• Biomass Gasification
• Thermochemical Production (Using a Heat-Driven Chemical Reaction to Split Water)
Electrolytic Processes
Water electrolysis uses electricity to split water into hydrogen and oxygen. Hydrogen produced via electrolysis can result in zero greenhouse gas emissions, depending on the source of the electricity used.
• Water Electrolysis (Splitting Water Using Electricity)
Photolytic Process
use light energy to split water into hydrogen and oxygen. Currently in the very early stages of research, these processes offer long-term potential for sustainable hydrogen production with low environmental impact.
• Photoelectrochemical Hydrogen Production (using Solar Power to Directly Split Water)
• Biological Hydrogen Production (photobiological water Splitting)
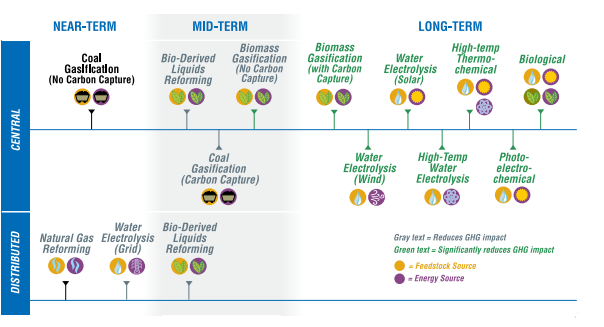
TECHNOLOGY SUMMARIES


Timeframes To Market
Some technology options necessarily appear at more than one location along this timeline as market readiness is affected by the specific feedstock, energy source, and production scale.

Thermal process
Distributed Natural gas reforming
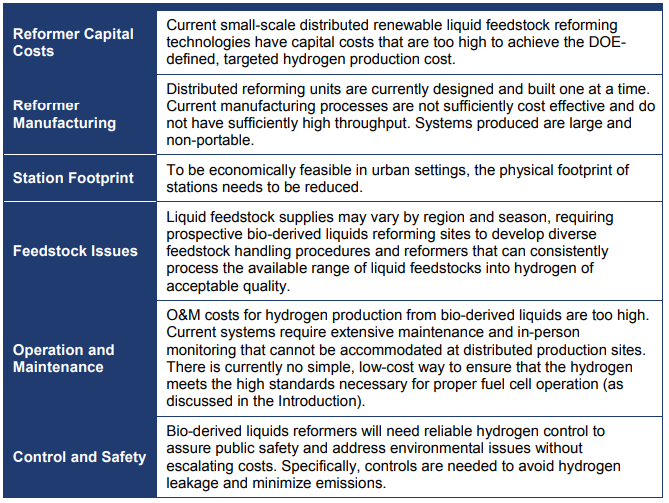
The distributed natural gas reforming pathway calls for producing hydrogen in distributed facilities via steam reforming of natural gas. Natural gas reforming is currently used on a large scale to produce much of the commercial and industrial hydrogen used today.



Biomasses derived liquids reforming
The bio-derived liquids reforming pathway calls for producing hydrogen in distributed facilities via gas-phase or aqueous-phase reforming. Hydrogen can be produced by reforming bio-liquids such as sugars, ethanol, or bio-oils. Reforming of ethanol and other bio-derived liquids is similar to natural gas reforming, but has several unique challenges, such as improved catalyst activity and durability. Some bio-derived liquids may also be cost effectively reformed at larger semi-central plants.


Biomass gasification
Biomass Gasification is a mature set of technologies that has the potential to supply the United States with a significant amount of low-cost hydrogen. Currently, these technologies are being applied to areas other than hydrogen generation, such as electrical power production, chemical production, and synthetic fuel production.
Combining feedstocks to offset challenges posed by either coal or biomass alone

Biomass Gasification
A majority of biomass like wood chip and agricultural/municipal waste can contain appreciable amounts of hydrogen. Gasification of such biomass can produce synthesis gas (syngas) which involves heating the organic material to above 700 °C under a controlled atmosphere of oxygen and/or steam as shown below. Gasification of biomass also produces useful by products such as ethanol and acetate.


Thermomechanical production
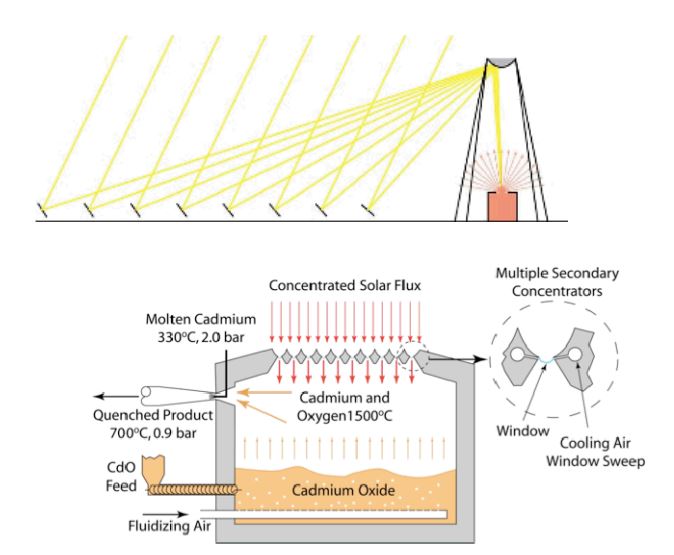
The thermochemical hydrogen (TCH) pathway calls for producing hydrogen in semi-central and central facilities via high-temperature thermochemical water splitting. TCH offers a potential technology for clean, sustainable hydrogen production.
TCH are being developed under the nuclear hydrogen initiative (NHI) using nuclear energy as the heat sources.
Concentrating solar energy to drive an efficient process


Electrolytic Processes
At times that renewable energy cannot be fed into the power grid due to network constraints or low demand, it could be supplied to electrolysers for production of hydrogen via electrolysis
Producing hydrogen from renewable power, especially when there is excess renewable electricity generation in the system, helps avoid curtailment and thereby improves the return on investment for VRE asset owners. Electrolysis can also enhance the reliability of power supply by providing flexibility services when needed. With increasing penetration of VRE resources such as solar PV and wind, large-scale storage solutions and grid stability management are becoming critical issues in system operation. Electrolysers can help integrate VRE into power systems, as their electricity consumption can be adjusted to follow wind and solar PV power generation, where hydrogen becomes a medium of storage for renewable electricity. Thus, electrolysers offer a flexible load and can also provide grid balancing services (upwards and downwards frequency regulation). The hydrogen produced can be further used in the industrial and transport sectors and as a fuel, or it can be stored and then converted back into electricity. Moreover, stored hydrogen may enable the efficient distribution of energy across regions, facilitating VRE consumption in areas where VRE generation is difficult or direct electrification of an end-use energy application.



Despite the fact that hydrogen can be produced in numerous ways, the most interesting but also promising part is the production of hydrogen through electrolysis of water.
In this process, the electrolysis breaks down water into hydrogen and oxygen by using electricity. If the electricity used, springs from renewable energy sources like wind or solar and the hydrogen produced is used in a fuel cell, then the entire energy process would create no net emissions. In this case, we would be talking about “green hydrogen”.
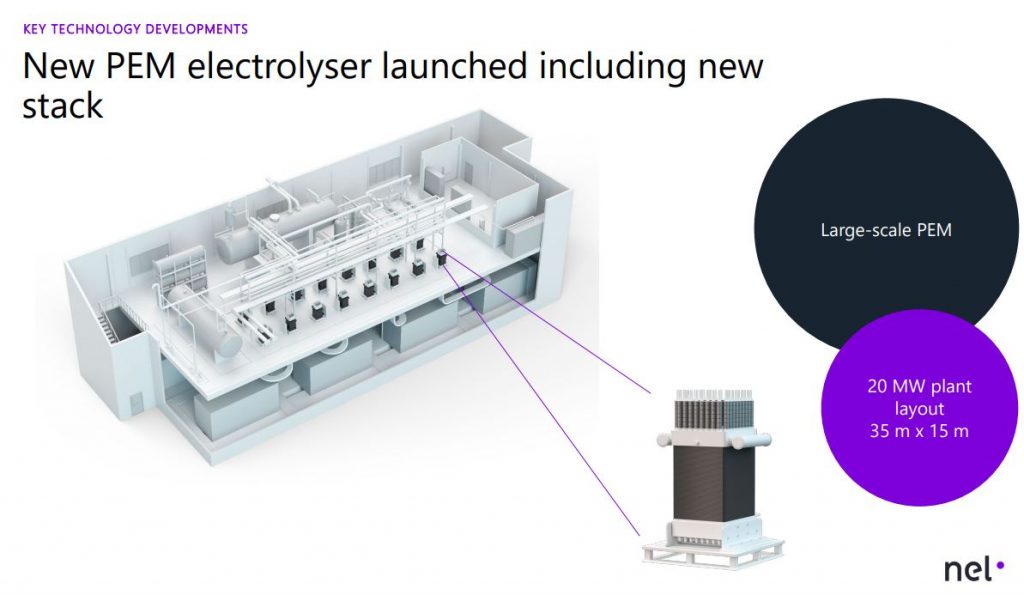
The electrolyser consists of a DC source and two noblemetal-coated electrodes, which are separated by an electrolyte. The electrolyte or ionic conductor can be a liquid, for example conductive caustic potash solution for alkaline electrolysis.
In an alkaline electrolyser the cathode (negative pole) loses electrons to the aqueous solution.
The water is dissociated, leading to the formation of hydrogen (H2) and hydroxide ions (OH –The charge carriers move in the electrolyte towards the anode. At the anode (positive pole) the electrons are absorbed by the negative OH – anions. The OH –anions are oxidised to form water and oxygen. Oxygen rises at the anode. A membrane prevents the product gases H2 and O2 from mixing but allows the passage of OH – ions. Electrolysers consist of individual cells and central system units (balance of plant). By combining electrolytic cells and stacks, hydrogen production can be adapted to individual needs.
Electrolysers are differentiated by the electrolyte materials and the temperature at which they are operated: low-temperature electrolysis (LTE), including alkaline electrolysis (AE), proton exchange membrane (PEM) electrolysis and anion
exchange membrane (AEM) electrolysis (also known as alkaline PEM), and high temperature electrolysis (HTE). The latter group most notably includes solid oxide electrolysis (SOE), but this is still at an advanced R&D stage and products are not yet commercially available. Once it reaches market maturity, its advantages are expected to include increased conversion efficiency and the possibility of producing a synthesis gas directly from steam and CO2 , for use in various applications such as synthetic liquid fuels.
High temperature electrolysis is particularly interesting when there is a source of heat next to the electrolyser (as it is often the case in industrial plants or) is more efficient economically than traditional room-temperature electrolysis. Indeed, some of the energy is supplied as heat, which is either free or cheaper than electricity, and also because the electrolysis reaction is more efficient at higher temperatures.
The choice of a given electrolysis technology depends on the use needs and the local context.
Hydrogen is like electricity in the sense that its use does not generate any emission.
Its carbon footprint is related to its production mode. In the case of hydrogen produced by electrolysis, its carbon footprint of hydrogen is directly related with the source of electricity. Hydrogen produced from carbon free renewable or nuclear electricity is therefore carbon free. Hydrogen produced with the grid mix has the same carbon intensity as the grid mix.
If the production of hydrogen can be the first objective of the separation process, it can also be that the separation process aims first at producing another molecule and produces hydrogen as a by-product.
Water electrolysis


Photolytic processes
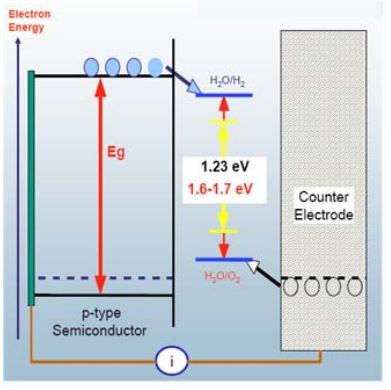

Photo Biological hydrogen production
The biological pathway calls for producing hydrogen using microorganisms. This is a long-term technology that will most likely be suitable for semi-central and central hydrogen production facilities.

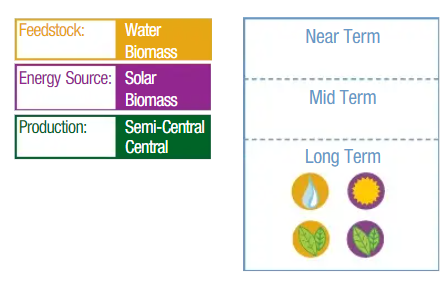
Other Biological Process
Photosynthetic Bacterial Production:
Sunlight is the driver for bacteria to break down organic material, thus releasing hydrogen. Purple, non-sulfur bacteria with a specialized enzyme can produce hydrogen gas when exposed to near-infrared light energy.
Dark Fermentative Production:
Bacteria can act on organic material and decompose it into hydrogen and other byproducts without the aid of sunlight. This process uses anaerobic bacteria that grow in the dark on carbohydrate-rich substrates. These bacteria break down biomass, which is relatively inexpensive, plentiful, and high in carbohydrate content. Researchers are working to identify specific strains of bacteria that can directly and efficiently ferment organic material (or cellulose) to hydrogen. Researchers will then develop mutations that selectively block the generation of waste acids and solvents that reduce ongoing hydrogen productivity.
Microbial-aided Electrolysis:
Microbial electrolysis cells use bacteria to efficiently extract energy from organic matter. As the bacteria decompose the organic materials, they produce a low voltage at the anode. Hydrogen is produced at the fully submerged
cathode with the input of a tiny amount of additional energy. Optimizing the environment to expedite this natural process could potentially produce hydrogen with much greater efficiency than standard electrolysis.
Combination:
Perhaps the most promising biological production pathway incorporates some or all of these technologies into a single system. This integrated approach could alleviate the need to overcome all barriers to individual technologies, as long as the overall system is cost-competitive. Integrated systems may use the byproducts of some production pathways as inputs to others in a nearly closed-loop system that produces hydrogen at each stage.

Microbial electrolysis cells use bacteria to break up acetic acid from plant waste and produce hydrogen gas using a bit of added electricity.
The process produces more than 250% more energy than the electricity required to extract it.
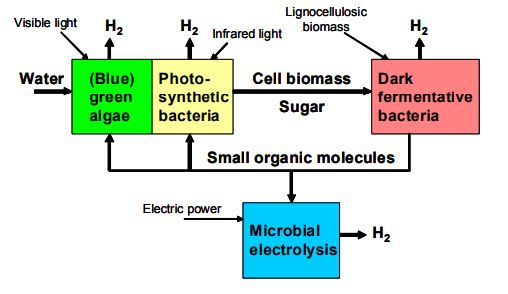
A system using multiple biological processes can provide internally generated feedstock and produce hydrogen at each step.
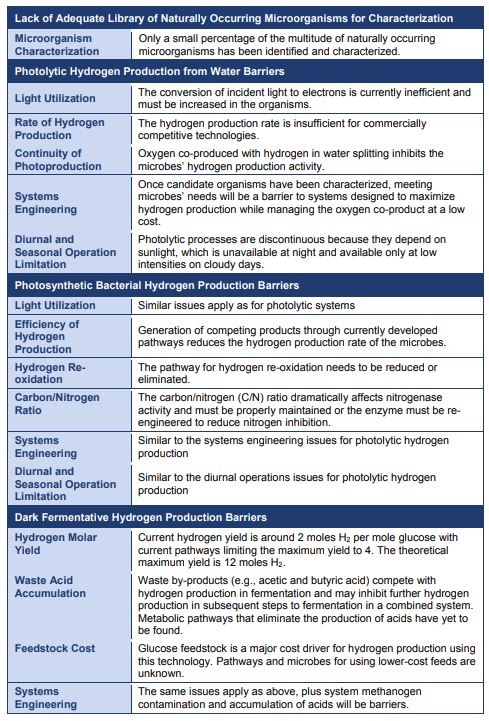
Next Step
Energy prices, supply uncertainties, and climate concerns are intensifying the need for diverse forms of energy from sustainable domestic resources.
Guided by the Hydrogen Production Roadmap, the FreedomCAR & Fuel Partnership and the DOE Hydrogen Program are working with researchers in national laboratories, universities, and industry to accelerate the development and commercial readiness of hydrogen production technologies and the supporting infrastructure.
The hydrogen initially available at consumer fueling stations will help to establish the markets, standards, and infrastructure. As research progresses, the technologies used to produce the hydrogen are expected to shift toward those that produce no net greenhouse gas emissions.
While some of the hydrogen production technologies now under development may be replaced by competing or improved approaches, a variety of production technologies are likely to find long-term use in regions that offer an abundance of their required feedstock and renewable energy resource. Fuel costs to consumers will gradually decrease as these technologies and the delivery infrastructure are optimized and grow to maturity.
Ultimately, hydrogen represents an important component of our national strategy to diversify energy resources. The Hydrogen Production Roadmap is helping to align public and private R&D priorities and technology investments to accelerate progress in bringing this clean, domestic energy carrier into widespread use.

