Author: Carlo Carniani – Senior Petroleum Engineer
Introduction
It is generally acknowledged that hydrogen gas is a promising resource for power production in the next future. Several publications are available reviewing the availability and use of hydrogen sources. While certain methods of hydrogen production are substantially known (e.g. electrolysis) and are expected to expand in the future, the possibility of exploiting native or geologic hydrogen is stil at its infancy. Several authors claim, on the basis of geological arguments, that hydrogen gas of natural origin may be abundant enough to be a significant energy source. The same authors claim that the reason why this resource is not presently available in large quantities might be a consequence of the fact that hydrogen is not actually searched with proper methods. (Zgonnik, 2020)
This possibility has pretty much to do with chemistry: the role of chemical analysis in hydrogen gas discovery is potentially decisive. According to the presently known discoveries, hydrogen gas is associated with other geological fluid sources: for example, geyser-like water sources like in Mali, block 25, Bourakébougou field (“Hydroma Inc. Block 25 Mali,”)).
Chemical analysis performed on site is crucial for identifying the presence of hydrogen and assess it quantitatively.
Moreover, since hydrogen gas is associated with other geological fluids (water, CO2, H2S, Hydrocarbons) a separation issue occurs as well.
Along with separation, gathering may also present different challenges from those the Oil&Gas industry is accustomed to face: rather than a limited number of high rate wells, it is more likely that native hydrogen has to be collected from a high number of very low rate sources.
Another important aspect in geological hydrogen production is storage: apart from long term storage in suitable underground reservoirs, analogously to what is currently happening with natural gas, it is conceivable that intermediate small scale storage steps will be required in an hypothetical future hydrogen gathering network.
A number of promising developments are possible out of recent results from research in materials science and fuel cells development. The purpose of this paper is giving a flavor of the ongoing chemical research on matters which may help in making native hydrogen a widespread energy source in the next future, without pretending of being exhaustive.
Phase behavior
Hydrogen gas or molecular hydrogen has been thoroughly studied by generations of chemists and physicists. Practically all of its properties are known, or are supposed to be known. In fact, several industrial applications have been developed across the time; the most important present applications are, in order of magnitude, ammonia production, petrochemicals, methanol production, summing up to 90% of the total hydrogen consumption (“HYDROGEN INDUSTRY APPLICATIONS: PAST, PRESENT, AND FUTURE.,” ).
All these activities allowed gathering a huge amount of data about hydrogen properties. However, the use of hydrogen for power generation raises certain challenges which still have to be addressed to; some of them are related to specific physical and thermal properties of hydrogen gas, which make it somewhat different from natural gas and affect the design and operation of a production system accordingly.
The impact of those properties (namely, boiling point, flammability, etc.) related to storage, and handling described in (C. Cappellani).
In this respect, two relevant features of hydrogen which is interesting to compare with natural gas ones are the Joule-Thomson (JT) effect and hydrate formation. The Joule-Thomson effect is a consequence of the fact that gases are not ideal. An ideal gas would expand without changing its temperature, while a real gas will cool down upon expansion.
This effect is largely exploited industrially, it is sufficient to think of gas separation and refrigeration. Hydrogen gas, instead, behaves in the opposite way: it warms up upon expansion. This effect may have consequences for separation and storage, namely issues in water-gas separation, which are discussed, for example, by Cappellani.
Another aspect of gas phase behavior which is little known but is not marginal at all is the formation of hydrates. Also known as clathrates, they are chemical compounds which form by inclusion of gas molecules (so called guest molecule) into a cage formed by water molecules (the host) held together by hydrogen bonds, as shown in the figure below.
Hydrogen hydrate structure red-white: water blue: hydrogen. From https://www.nature.com/articles/srep05606/figures/2
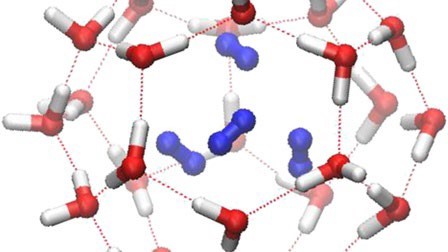
Gas hydrate looks like ice or frost and form when gas and water get in contact at certain temperature and pressure conditions, which vary upon the nature of the guest substance.
There are many kinds of hydrate compounds: in the oil and gas industry, methane hydrate are known to be often an issue in hydrocarbon production, since they may obstruct or plug gas-bearing flowlines.
On the other hand, naturally occurring methane hydrate present on ocean’s floor has been considered as a potential energy source. Hydrogen gas is also capable of forming hydrates, which may contain from 3 to 6% weight of H2, depending on the crystal structure. This aspect will be discussed later in this paper, along with other storage materials opportunities offered by research.
Storage, Gathering and Separation
A number of hydrogen storage techniques are available today, which are described by (C. Cappellani) (ibidem). Although several systems are commercially ready, opportunities for improvement still exist. For example, liquid hydrogen storage requires a considerable energy cost and strict safety procedures, which are hardly compatible in a situation similar to Bourakébougou case. By the way, reduction of cost and simplification of operating conditions justifies the undergoing research effort on new storage techniques and materials. Under a chemical or materials research perspective, several promising areas of investigation are being explored at present. A hydrogen storage material should possess these properties: high gas retaining capacity, which means high in-situ gas density, as long as reversible adsorption/desorption at ambient conditions with a proper kinetics.
These requirements are especially stringent for automotive applications. DOE (“Hydrogen Storage,”) set a target for storage materials in terms of amount of stored hydrogen: a material is expected to be able to take up 5.5% wt or 40 Kg H2/m3, in order to be deployed for practical purposes. It is known since a long time that palladium may take up significant amounts of hydrogen. The review by Adams (Adams and Chen, 2011). provides an overview about the present and future uses of Pd-based materials in a hydrogen-based economy. While Pd itself is probably too expensive for distributed hydrogen storage, it could be a fundamental component in cheaper, carbon-based (graphite) storage materials. In this case, Pd particles would act as catalysts of molecular hydrogen dissociation, facilitating the successive adsorption of hydrogen atoms by the carbon/graphite substrate. Another field of research in hydrogen storage materials are metal hydrides (Zuettel, 2004) in this respect, four groups are identified, depending on the nature of the active chemical bond in the substance: conventional hydrides, complex hydrides, chemical hydrides, and sorbent systems (e.g. Metal Organic Frameworks, MOF) (“Transportation and Storage,”).
The latter deserve a little explanation: MOF are high surface area materials made of metal clusters connected by organic molecules. The combination of inorganic components and organic molecules allows the synthesis of a wide range of materials with tailored properties, making them very promising for future applications The list of possible candidates as materials for hydrogen storage also includes nanoporous materials Research groups are investigating several different nanostructures made of carbon, graphene, silicon carbide doped with other atoms, which seem promising for future applications ((Yang et al., 2010)). Another unconventional material for H2 storage, as anticipated in a previous section, would be gas hydrate (Antonin Chapoy, and et al.,) (Gupta et al., 2021). In this case, the objective is obtaining a significant adsorption of H2 into the hydrate at the lower possible pressure. Hydrates made of pure H2 do not meet such objectives. Mixed hydrate with Argon or nitrogen, instead, reached H2 uptakes up to 5% wt or 48 Kg/m3 at moderate pressures and temperatures around ambient. Although still far from DOE targets, such results indicates a way for possible future developments.
Membrane separation
Separation of gases by membranes is a consolidated industrial process ((Adhikari and Fernando, 2006).) with several applications. Membrane technologies may have a considerable impact on the realization of hydrogen collecting systems from natural sources: with respect to those processes presently used for hydrogen gas separation, namely Pressure Swing Adsorption (PSA) or cryogenics, membranes can be adapted, in principle, to small size plants with reduced footprint and costs.
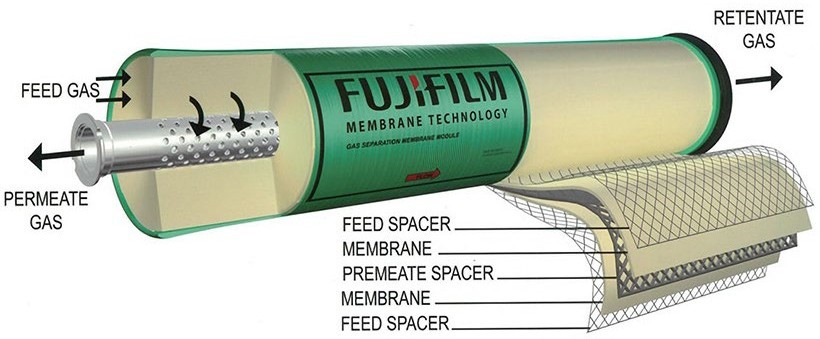
Concerning specific studies of hydrogen separation, (Adhikari and Fernando, 2006) indicates five types of membranes, depending on the material class they are made of: dense polymer membranes, microporous ceramics, dense metallic membranes, porous carbon and dense ceramics. Key factors for membrane efficiency are separation selectivity, hydrogen flux and sensitivity to poisoning agents. Dense polymer membranes work at room temperatures or lower and perform with low selectivity and H2 flux. The best sensitivity and flux are obtained with Pd-based dense metallic membranes, that are sensitive to H2S, CO and HCl. Dense ceramics also show good selectivity, but are sensitive to H2S and may have stability issues in CO2-rich gases. Microporous ceramics, made of combinations of silica, zirconia and similar oxides, display intermediate selectivity and flux, coupled with resistance to poisoning agents. They perform better at high temperatures and may have stability issues in the presence of water.
By the way, a number of possible solutions exist, which may be fit to different composition of the hydrogen-containing gas to be exploited and the conditions at which it is going to be produced.
Chemical Analysis Tools
The identification of hydrogen in geological fluids has mostly been obtained, so far, with the use of chemical analysis, typically by portable devices incorporating miniaturized sensors ((Moretti et al., 2021).). The advantage of such instruments are the ease of use, low cost and accuracy sufficient for several monitoring purposes. The variety of available sensors makes possible the identification of several gases at once. A possible disadvantage of such devices is that they are designed to search for low levels of chemicals, in the order of 2000 ppm of H2 or less, with a sensitivity of 5 ppm (from spec of a commercial device).
A class of portable analytical instrument is also available, namely gas-chromatography-mass spectrometers (GC-MS), which identify and quantify different chemical species in complex mixtures within a wiide range of concentrations. Typically, GC-MS equipment is bench-scale laboratory instrumentation; however, miniaturized devices were designed to meet the needs of space exploration ((Snyder et al., 2016)). The range of available instrumentation now spans from handheld commercial equipment, whose main application is monitoring for environmental, health or security, to small size devices for aerial survey of volcanic plumes by means of equipment mounted on unmanned aerial vehicles ((Diaz et al., 2015)).
The performance of portable GC-MS has reached very good levels in terms of sensitivity and accuracy in the analysis of complex mixtures: for example, a prototype compact GC-MS set-up designed for Space Shuttle usage reached a 12ppm detection level of H2 with an accuracy of 7%. ((Ottens et al., 2002))
In principle, the possibility of analyzing many substances at a time opens the way to the search for correlations which may provide useful clues for exploration, in much of the same way that geochemical investigations is used in upstream O&G.
By the way, we may expect that natural hydrogen exploration will increase significantly the number and size of hydrogen accumulations by an extensive use of GC-MS portable instrumentation and, possibly, other small size analytical devices as well.
Comparison between the upstream oil/gas and the native hydrogen production system concepts.
A high level description of an hydrocarbon production system relies upon the following macro- elements:
- the reservoir: a large size, underground accumulation, with identifiable boundaries
- the well: a fixed structure designed to connect reservoir to surface. A major concern with well design is the control of hydrocarbon flow, both for safety of operation and maximization of
- the gathering network: a system of pipes connecting wells to the processing and storage plant
- Surface facilities: this system includes vessels for separation of gas from liquid components, control and measurement devices, intermediate storage tanks and delivery equipment to larger networks or refining plants
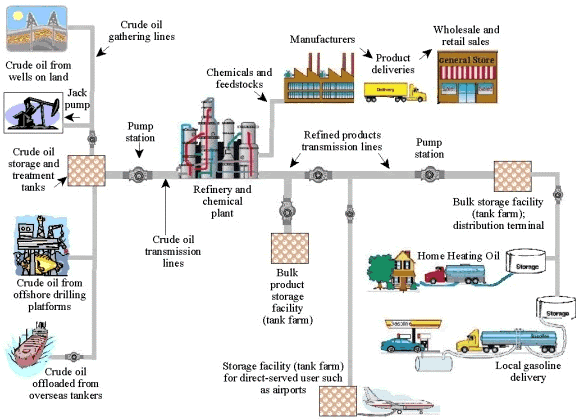
The above sketch describes a basic onshore system; in an offshore environment, the same elements are present, with obvious differences which make their realization more complex and expensive. The main common feature among different O&G production systems is the capability of handling large volumes of hydrocarbons.
A native hydrogen production system might be somewhat different. First of all, the hydrogen- bearing “reservoir” is going to be different in size, lithology, morphology and fluid content, according to the present knowledge. In the case of Mali (Bourakébougou field) the reservoir shows several analogies with a conventional gas one, letting apart the fundamental fact that the rock is not sedimentary; for example, geophysical data indicate the presence of a trap. The most abundant fluid in the accumulation, however, is not hydrogen but water. The size of the hydrogen in place is significantly smaller than most of commercially developed hydrocarbon reservoirs. The depth is also smaller with respect to most presently active oil and gas reservoirs; a consequence of this is that hydrogen comes to surface at a pressure which is low but sufficient high to fill a GPL-storage vessel, where the gas is separated and transferred to a fuel cell for immediate use.
Of course, it is far from granted that this configuration is going to be the standard across possible future geological hydrogen developments. However, if an assumption is made that this kind of asset will be replicated, a few remarks can be made:
- reservoirs are expected to be small and spread over the investigation area
- wells will possibly be less challenging with respect to ordinary upstream wells, because of a shorter depth and less fluid
- gathering will be possibly a major issue, since transportation of small volumes of hydrogen over long distances might not be cost effective. On the contrary, power production at wellsite would probably be a better alternative. In those cases where energy cannot be used locally, an alternative option would be the accumulation of hydrogen in transportable units to be deployed
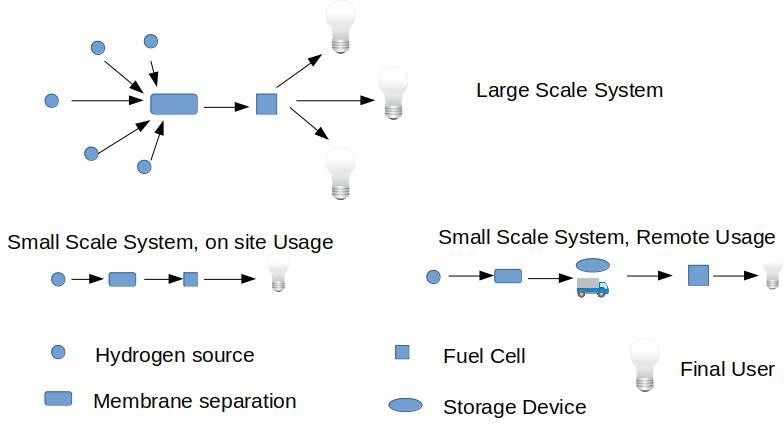
In this respect, research on unconventional materials plays an important role for the realization of intermediate small size storage devices for collection and distribution of hydrogen to the final destination, that, in most cases, is a fuel cell system.
Fuel cells
Fuel cells (FC) are energy production devices based on hydrogen; they are available in a wide range of dimensions and configurations, which make them suitable for applications ranging from massive power production to automotive. They are possibly going to be the pivot of a future H2-based distributed energy production system. FCs have been known and used for a long time, for example in NASA’s Apollo missions. They consist in an electrochemical device which converts chemical energy into electric power. In this sense, fuel cells and batteries have many similarities. The difference between a battery and a fuel cell is that the former stores the chemical energy in its interior, while the energy storage is external in the latter. A schematics of a fuel cell working principle is shown below:
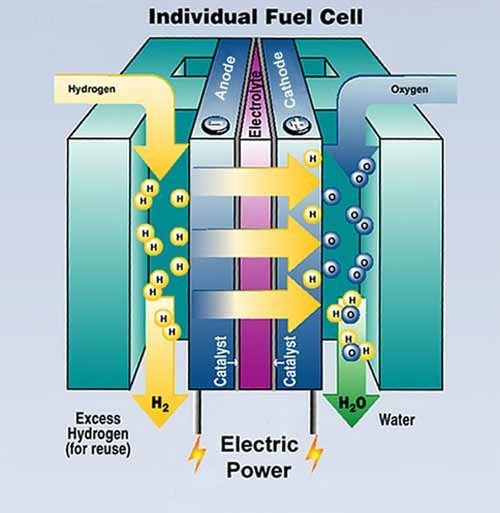
Basically, a chemical reaction set in the cell, converting hydrogen and oxygen (from air) into water and producing energy at the same time, in the form of an electric current.
There are several kinds of fuel cells ((“Hydrogen and Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan,”)) according to the materials which they are made of:
Alkaline (AFC): this is the oldest and still more efficient type of FC with a capacity of power generation up to 100 kW. NASA used this type of FC in Apollo and Space Shuttle missions. H2 and O2 are supplied to the cell by carbon electrodes immersed in a concentrated alkali solution, which acts as conducting electrolyte. A suitable metal catalyst speeds up the reaction between hydrogen and oxygen to produce water and generate power. In certain AFC the electrolyte solution is replaced by a solid state, polymer based anion exchange membrane. A major drawback of AFC is the necessity to use pure oxygen or highly purified air, in order to avoid poisoning by carbonate deposits formed by reaction of the electrolyte with CO2 in the air.
Proton Exchange Membrane (PEM; also Polymer Electrolyte Membrane): they consist of a polymer membrane electrolyte coupled with electrodes containing platinum particles acting as a catalyst. They operate at 80 to 100 °C and have a small footprint and weight, making them suitable for automotive applications. The use of PEMFC is presently growing faster than the other FC types.
Phosphoric Acid (PAFC): this was the first commercial type of FC; therefore, its technology is more mature compared to other FC types. The electrolyte used is phosphoric acid, while the electrodes are made of carbon and silicon carbide, supporting a finely dispersed platinum powder (catalyst). Their main use is power generation in buildings, proving to be reliable. Advantages are easier management of water and tolerance to impurities in hydrogen. Drawbacks are the cost (less energy per unit weight compared to other FC) and control of vapors containing phosphoric acid.
Molten Carbonate (MCFC): they use a molten salt carbonate electrolyte and operate at 650 ºC. This makes possible the thermal conversion (reforming), of several hydrogen sources like natural gas, methanol, ethanol, biogas, and coal. No noble metal catalyst is used, which reduces the cost. Heat recovery is possible, which may increase the total efficiency. The disadvantages are the need to control corrosion, the time required to reach the operating temperature and slow response to changes in electricity demand. The best application for MCFC is constant electricity supply in large utilities.
Solid Oxide (SOFC): they are made of a ceramic material which acts as a solid electrolyte. Since SOFCs are solid, they can be shaped as tubes and are not limited to made entirely of solid materials, they are not limited to the parallel plate assembly. SOFC work at temperatures above 800 °C and may accept fuels different from hydrogen, which is generated internally by reforming, in a way similar to what happens in MOFC.
The different classes of fuel cells are suited to different applications: for example, PAFC, MCFC and SOFC are most suited for distributed generation, while PEM and AFC have been mostly applied in specialty vehicles (e.g. submarines, buses).
Although fuel cells are conceived to use hydrogen as a fuel, different chemical substances have also been employed, like methanol, which is “hydrogen dense”. In this case, methanol is either converted to hydrogen by steam reforming (indirect fuel cell) or into the fuel cell itself (direct methanol fuel cell) thanks to a suitable catalyst.
The operation of a fuel cell requires a catalyst or high temperature or a combination of both. A balance has to be found between the catalyst cost when working at low temperatures and the cost of waste heat and corrosion when the fuel cell works at high temperatures, as it is the case for SOFC.
Research is being carried out in industry and academia to obtain devices working at low temperatures and using inexpensive catalysts (see, for example (Litzelman and Lemmon, 2015)., which reviews research oriented to fuel cells for automotive applications).
A few results are listed here, according to the various section of a typical fuel cell assembly.
The Membrane-electrode assembly is the layer of the fuel cell where hydrogen and oxygen get in contact first, it contains the catalyst and its morphology affects the effectiveness of it. Interesting results are being obtained using nano-structured thin films to support the platinum catalyst, so that higher efficiency and durability may be obtained.
The Polymer Exchange Membrane (also known as Proton Exchange Membrane) is being improved by the use of composite materials and reduction of its thickness to less than 50um, which results in reducing impedance, increasing conductivity and improving thermal and mechanical stability.
The Catalyst system is a major source of costs in a fuel cell. Presently, there are two main research directions for the development of new catalysts:
- use alloys of platinum with cheaper metals and play with particles size to obtain the desired performance
- develop platinum-free catalysts; for example, manganese and nitrogen doped carbon systems (Mn-N-C) seem to have interesting performances at a much lower cost compared with platinum
In a SOFC cell, it would be desirable reducing this temperature in a range between 600-800°C, with advantages in cost and ease of operation. In this respect, several challenges have to be overcome: slow reaction kinetics, poisoning by metal impurities and material degradation by CO2 or humidity. Research is in progress ((Ndubuisi et al., 2022).) for developing SOFC with special ceramics which is giving promising results in this respect.
A major subject in fuel cell operation is corrosion. Depending on the type of fuel cell, several corrosion issues may occur which require different approaches for achieve control over them.
For example, surfaces can be protected by coatings or surface treatments (Antunes et al., 2010) including such techniques like Plasma or Chemical Vapour Deposition (Fan et al., 2021).
In addition, other approaches (Perry et al., 2006) have still to be investigated: for example, combination of surface treatments with doping by suitable elements and protective coatings.
In SOFCs corrosion occurs (Yang et al., 2017) because of the unavoidable presence of certain contaminants, like chromium or silica, along with water vapor and CO2 coming from air. At present, the solution of such issues is searched through doping of the cathode material to achieve better corrosion resistance and supplying dry air to the fuel cell.
By the way, corrosion monitoring is likely to be a very important factor in the operation of a distributed energy system based on fuel cells fed by native hydrogen sources. Corrosion monitoring and control may result in operating expenditure and complex logistics. Therefore, monitoring methods may play a very important role in the massive deployment of fuel cells. While most of the techniques described in the quoted references are suited for research laboratories, the underlying principles may possibly suggest methods better fitted to field monitoring. For example, Antunes (Antunes et al., 2010) points out that several corrosion mechanisms occurring in fuel cells can be monitored by chemical analysis of specific chemical species that are released during the corrosion process. This situation opens the way to the implementation of chemical monitoring techniques to support fuel cell operation in a distributed energy delivery system.
In this respect, expertise and technologies from other industries, including O&G, may possibly prove useful for a proper management this chemistry-related issue.
Concluding remarks
Hydrogen from natural sources is a potentially important source of energy for the future. However, the exploitation of native hydrogen poses a number of challenges at all stages of the cycle, from discovery to delivery at the final client.
A possible scenario for future geological hydrogen use is the realization of a network of small production sites equipped for direct power delivery in the surroundings and storage in transportable small scale devices.
The feasibility of such a scenario depends on the availability of suitable technologies: sensitive detection devices for exploration, compact separation systems, high capacity storage materials, cost- effective fuel cells assemblies, solutions for asset integrity and so on.
Research in chemistry and materials science is already testing a number of solutions which will play a key role in the future deployment of native hydrogen production systems.
References
Adams, B.D., Chen, A., 2011. The role of palladium in a hydrogen economy. Mater. Today 14, 282– 289. https://doi.org/10.1016/S1369-7021(11)70143-2
Adhikari, S., Fernando, S., 2006. Hydrogen Membrane Separation Techniques. Ind. Eng. Chem.
Res. 45, 875–881. https://doi.org/10.1021/ie050644l
Antonin Chapoy, , and, Ross Anderson, Bahman Tohidi, n.d. Low-Pressure Molecular Hydrogen Storage in Semi-clathrate Hydrates of Quaternary Ammonium Compounds | Journal of the American Chemical Society [WWW Document]. URL https://pubs.acs.org/doi/10.1021/ja066883x (accessed 7.6.22).
Antunes, R.A., Oliveira, M.C.L., Ett, G., Ett, V., 2010. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrog. Energy 35, 3632–3647. https://doi.org/10.1016/j.ijhydene.2010.01.059
Arkin, C.R., Griffin, T.P., Ottens, A.K., Diaz, J.A., Follistein, D.W., Adams, F.W., Helms, W.R., 2002. Evaluation of small mass spectrometer systems for permanent gas analysis. J. Am. Soc. Mass Spectrom. 13, 1004–1012. https://doi.org/10.1016/S1044-0305(02)00422-1
- Cappellani, n.d. Hydrogen Underground Storage: Status of Technology and Perspectives. Comparison of Fuel Cell Technologies, n.d. 1.
Diaz, J.A., Pieri, D., Wright, K., Sorensen, P., Kline-Shoder, R., Arkin, C.R., Fladeland, M., Bland, G., Buongiorno, M.F., Ramirez, C., Corrales, E., Alan, A., Alegria, O., Diaz, D., Linick, J., 2015. Unmanned Aerial Mass Spectrometer Systems for In-Situ Volcanic Plume Analysis. J. Am. Soc. Mass Spectrom. 26, 292–304. https://doi.org/10.1007/s13361-014-1058-x
Fan, L., Tu, Z., Chan, S.H., 2021. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 7, 8421–8446. https://doi.org/10.1016/j.egyr.2021.08.003
Germain, J., Fréchet, J.M.J., Svec, F., 2009. Nanoporous polymers for hydrogen storage. Small Weinh. Bergstr. Ger. 5, 1098–1111. https://doi.org/10.1002/smll.200801762
Gupta, A., Baron, G.V., Perreault, P., Lenaerts, S., Ciocarlan, R.-G., Cool, P., Mileo, P.G.M., Rogge, S., Van Speybroeck, V., Watson, G., Van Der Voort, P., Houlleberghs, M., Breynaert, E., Martens, J., Denayer, J.F.M., 2021. Hydrogen Clathrates: Next Generation Hydrogen Storage Materials. Energy Storage Mater. 41, 69–107. https://doi.org/10.1016/j.ensm.2021.05.044
Hydrogen and Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan [WWW Document], n.d. Energy.gov. URL https://www.energy.gov/eere/fuelcells/articles/hydrogen-and-fuel-cell-technologies-office- multi-year-research-development (accessed 7.6.22).
HYDROGEN INDUSTRY APPLICATIONS: PAST, PRESENT, AND FUTURE. [WWW
Document], n.d. URL https://wha-international.com/hydrogen-in-industry/
Hydrogen Storage [WWW Document], n.d. Energy.gov. URL https://www.energy.gov/eere/fuelcells/hydrogen-storage (accessed 10.10.22).
Hydroma Inc. Block 25 Mali, n.d.
Litzelman, S.J., Lemmon, J.P., 2015. The Promise and Challenges of Intermediate Temperature Fuel Cells. ECS Trans. 68, 39–47. https://doi.org/10.1149/06801.0039ecst
Moretti, I., Prinzhofer, A., Françolin, J., Pacheco, C., Rosanne, M., Rupin, F., Mertens, J., 2021. Long-term monitoring of natural hydrogen superficial emissions in a brazilian cratonic environment. Sporadic large pulses versus daily periodic emissions. Int. J. Hydrog. Energy 46, 3615–3628. https://doi.org/10.1016/j.ijhydene.2020.11.026
Ndubuisi, A., Abouali, S., Singh, K., Thangadurai, V., 2022. Recent advances, practical challenges, and perspectives of intermediate temperature solid oxide fuel cell cathodes. J. Mater. Chem. A 10, 2196–2227. https://doi.org/10.1039/D1TA08475E
Onboard Hydrogen storage for Light Duty Fuel Cell Vehicles, n.d.
Ottens, A.K., Harrison, W.W., Griffin, T.P., Helms, W.R., 2002. Real-time quantitative analysis of H 2 , He, O 2 , and Ar by quadrupole ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 13, 1120–1128. https://doi.org/10.1016/S1044-0305(02)00431-2
Perry, M.L., Patterson, T., Reiser, C., 2006. Systems Strategies to Mitigate Carbon Corrosion in Fuel Cells. ECS Trans. 3, 783–795. https://doi.org/10.1149/1.2356198
Snapshot, n.d.
Snyder, D.T., Pulliam, C.J., Ouyang, Z., Cooks, R.G., 2016. Miniature and Fieldable Mass Spectrometers: Recent Advances. Anal. Chem. 88, 2–29. https://doi.org/10.1021/acs.analchem.5b03070
Transportation and Storage, n.d. Hydrog. Portal. URL https://hydrogen- portal.com/technologies/transportation-and-storage/ (accessed 10.10.22).
Yang, J., Sudik, A., Wolverton, C., Siegel, D.J., 2010. High capacity hydrogen storage materials: attributes for automotive applications and techniques for materials discovery. Chem Soc Rev 39, 656–675. https://doi.org/10.1039/B802882F
Yang, Z., Guo, M., Wang, N., Ma, C., Wang, J., Han, M., 2017. A short review of cathode poisoning and corrosion in solid oxide fuel cell. Int. J. Hydrog. Energy 42, 24948–24959. https://doi.org/10.1016/j.ijhydene.2017.08.057
Zgonnik, V., 2020. The occurrence and geoscience of natural hydrogen: A comprehensive review.
Earth-Sci. Rev. 203, 103140. https://doi.org/10.1016/j.earscirev.2020.103140
Zuettel, A., 2004. Hydrogen storage methods. Naturwissenschaften 91, 157–172. https://doi.org/10.1007/s00114-004-0516-x

