Author: Carlo Cappellani – Senior Geoscientist
INTRODUCTION
The global energy sector is transforming and hydrogen (the most energy-rich gas) is likely to play an increasingly prominent role as a clean energy carrier. Many countries have identified hydrogen as a key pathway to decarbonise their transport, industry processes, heating and energy storage sectors.
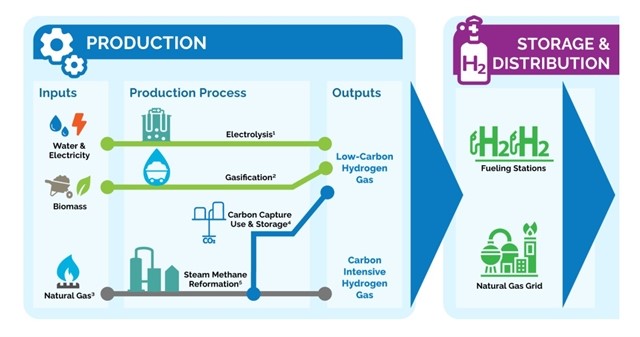
Hydrogen is almost exclusively manufactured for industrial use, with around 840 Bm3 per year being produced worldwide (Wood Mackenzie 2021).
It can be produced artificially via a variety of different pathways and the primary methods for production of hydrogen with low carbon emissions being
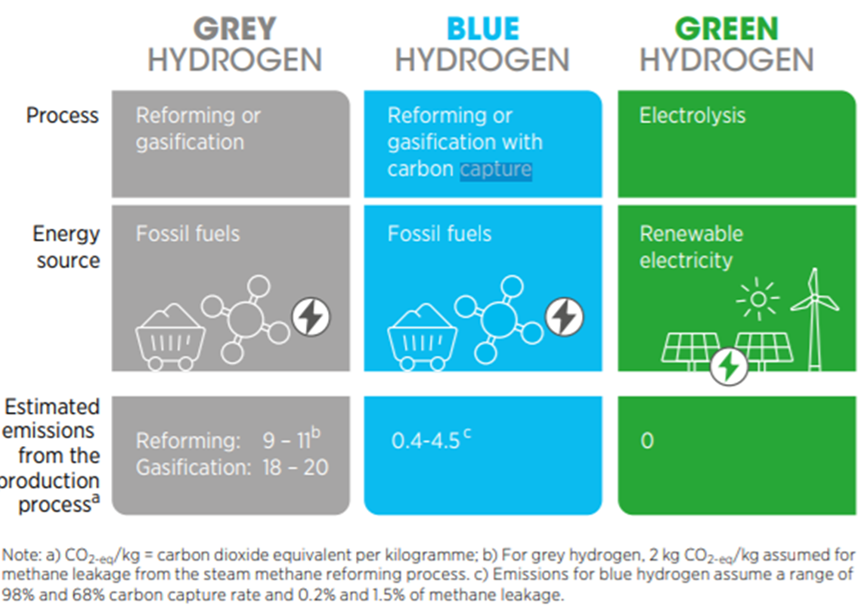
- water electrolysis using renewable energy (green hydrogen)
- steam reformation of natural gas paired with carbon capture and storage (CCS; blue hydrogen)
- coal gasification combined with CCS (also blue hydrogen).
Note:
- the majority of produced hydrogen originates from hydrocarbon-based feedstock without CCS (grey hydrogen) since the economics for the electrolytic production of green hydrogen (0.1% of total H2 production) requires improvement (Wood Mackenzie 2021).
- For a large-scale hydrogen industry to develop, hydrogen storage is key and hydrogen storage in salt caverns is considered the most promising approach for large-scale seasonal storage (HyUnder 2013; Caglayan et al. 2020).
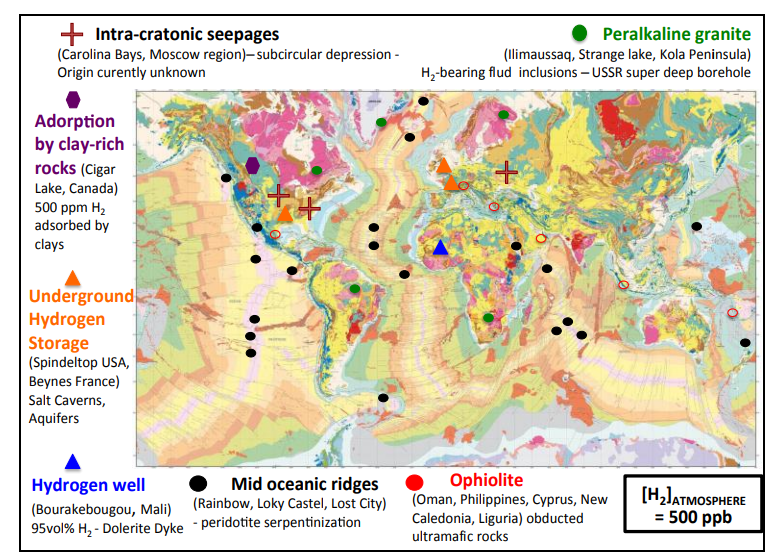
Hydrogen is seemingly ubiquitous in the subsurface, being classified as natural or native or geological.
Geological hydrogen could revolutionise the low-carbon future as it can be exploited economically
- removing the need for clean water – used during green hydrogen electrolysis
- eliminating the need for expensive Carbon Capture and Storage (CCS) associated with blue hydrogen.
The processes that create natural hydrogen are not fully understood and the discovery of this potentially renewable resource raises several questions related to:
- Commercial accumulations
- Method of exploitation
- Legal implications of exploring for hydrogen
- Costs of production
- Can it decarbonise and compete with existing (grey and blue) hydrogen feedstock, or even green hydrogen?
- Hydrogen storage in the subsurface?
It is found in a large range of geological settings
- oceanic and continental crust
- volcanic gases
- hydrothermal systems
The present known sources of natural hydrogen seem to be abiotic, however, discussions highlighted that natural hydrogen can be considered as both biogenic or abiotic, because source interpretations can be subjective, and there is still much to learn about hydrogen and microorganisms.
GEOLOGICAL OCCURRENCES, SOURCES AND SINKS OF NATURAL HYDROGEN
More specifically, natural hydrogen is present in a range of geological environments such as crystalline basement, volcanic, ultramafic and peralkaline igneous complexes, geothermal and mineral systems, graphite, evaporite deposits and anoxic sediments, as well as in conventional and unconventional gas and oil fields, and coal seam gas (Gregory et al. 2019).
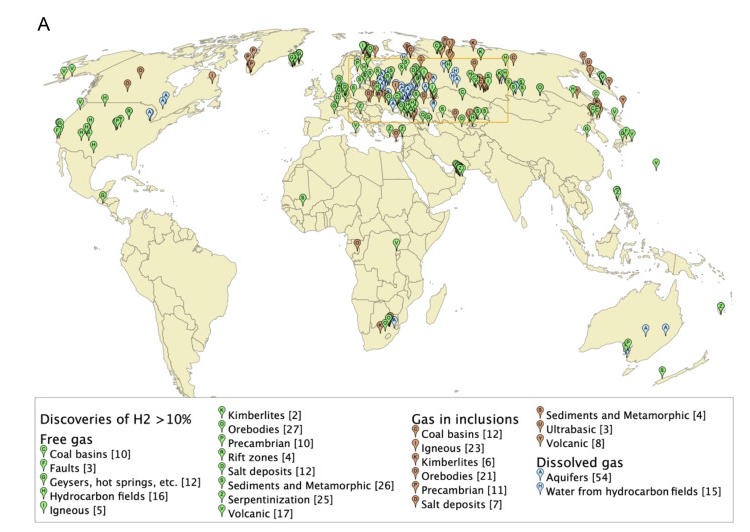
Since H2 is mobile and chemically active, gases stored for millennia in reservoir rocks may require a continuous replenishment of H2 from disparate source(s) (Dubessy et al. 1989; Zgonnik 2020).
H2 contents within gas and oil fields, in general, are commonly lower than surrounding rocks suggesting a chemical reaction between H2 and the emplaced organic matter (Zgonnik 2020).
Natural H2 sources can be classified natural into two groups (Zgonnik, 2020):
- primary hydrogen stored within the earth’s hydridic core and mantle (Larin 1993; Walshe et al. 2005; Walshe 2006)
- secondary hydrogen generated from chemical reactions within the mantle and crust.
Natural H2 can be regarded as having either an abiogenic (Reeves and Fiebig 2020) or a biogenic source, of which the latter includes both thermogenic (Tissot and Welte 1984; Hunt 1996) and microbial (Nandi and Sengupta 1998; Hallenbeck and Benemann 2002) processes.
The isotopic signature of H2 can be used to help determine its origin.
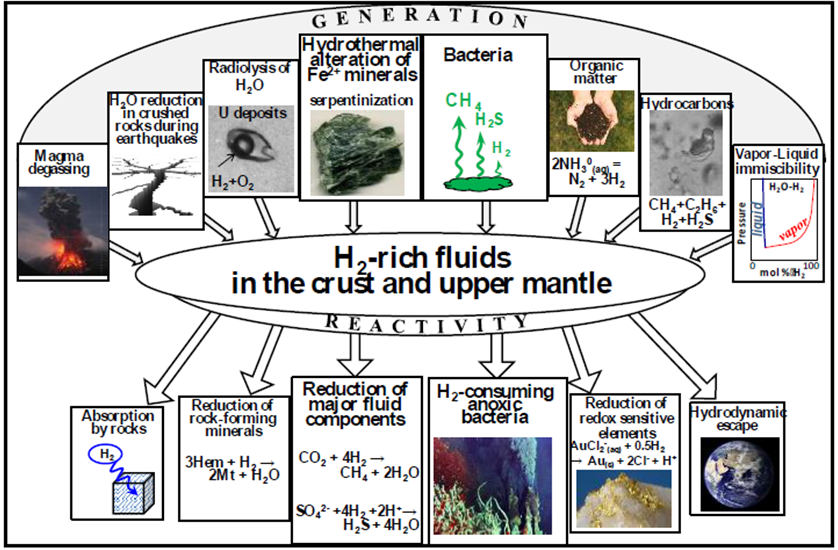
SOURCES OF NATURAL HYDROGEN
Primary source of natural hydrogen
Hydrogen and helium (He) on earth have their roots in the formation of the universe and the solar system.
Primordial H2 was initially captured then dissociated to form a hydritic core and lower mantle during the formation of the earth (Rumyantsev 2016), estimated to be some 4.54 billion years ago 50 million years (National Geographic 2021). Some consider primary H2 resources infinite for human usage, if only accessible (Larin 1993; Zgonnik 2020; Moretti et al. 2021b).
Ultramafic rocks dominate the mantle and fluids in the lower mantle are likely to contain methane (CH4), H2 and water (H2O), whereas in the upper mantle they comprise carbon dioxide (CO2) and water. This primordial source of hydrogen is in the form of stored primary metal hydride and is considered the dominant form of hydrogen on earth (Zgonnik 2020).
These metal hydrides release H2 through processes resulting in the continuous and intermittently violent injections into the upper mantle and crust. Such large-scale fluid migration can have major impacts on all earth processes within the upper lithosphere, biosphere, hydrosphere and atmosphere (Walshe et al. 2005; Walshe 2006).
Secondary sources of natural hydrogen
Secondary (abiogenic) sources of natural hydrogen are potentially the largest yet-to-find resources, although exploration for petroleum and the recovery of biogenic natural gas (with non-commercial H2) is the largest currently exploited resource.
Abiogenic H2 might influence crustal redox chemistry that control deposition of ore mineralisation (Walshe et al. 2005; Zgonnik 2020), and hence the analysis of gases from mines would be beneficial (Boreham et al. 2021).
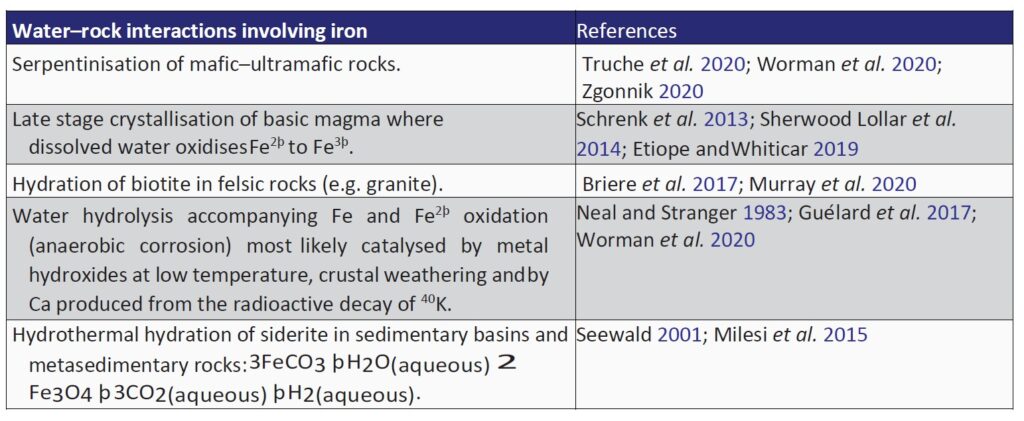
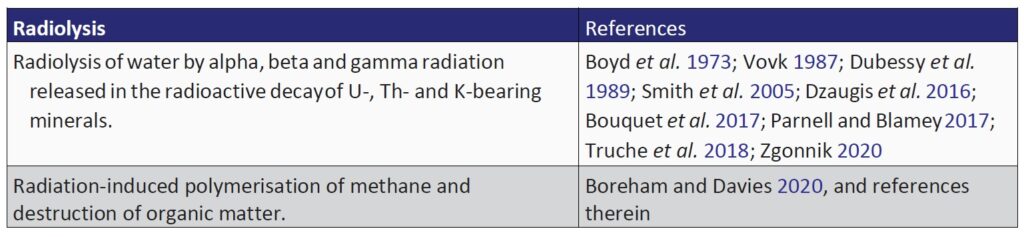
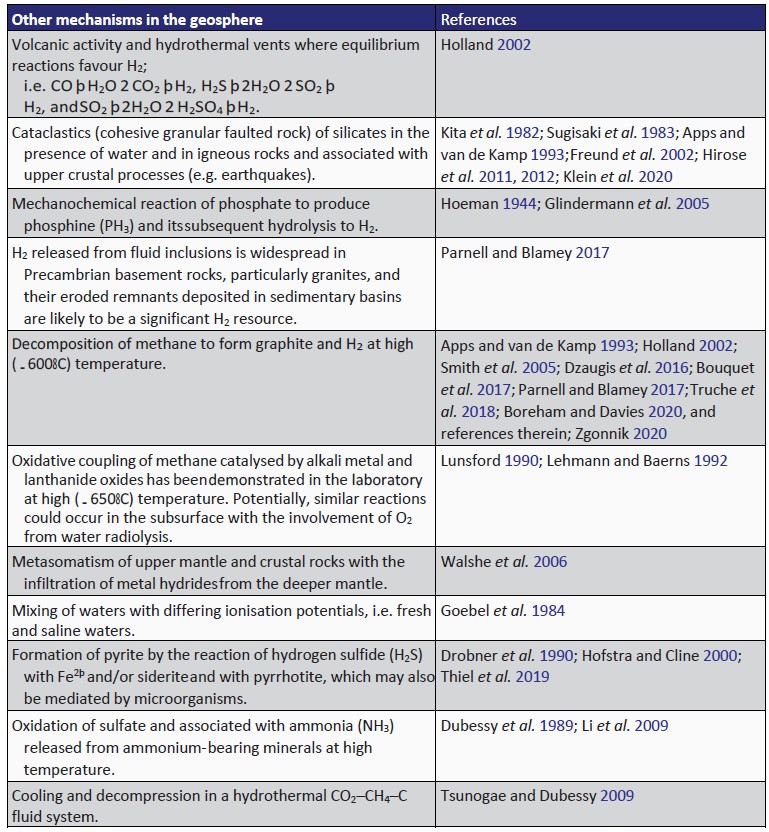
Numerous abiogenic sources of natural H2 are summarised in Table 1, with serpentinisation, radiolysis and volcanic and hydrothermal vents being considered some of the most important geological processes
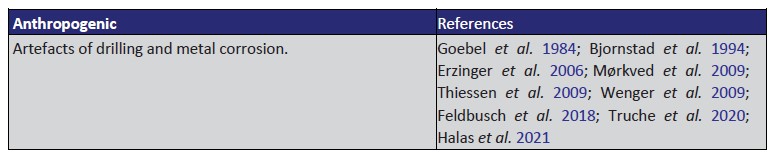
Serpentinisation is the most studied reaction pathway to H2 in the subsurface and it occurs with the circulation of hydrothermal fluids through mafic–ultramafic rocks on the seafloor at deep- sea hydrothermal vents and in continental ophiolites.
Serpentinisation can occur at temperatures from just above ambient to 330–400 C, depending on pressure (Evans et al. 2013; Mayhew et al. 2013; McCollom and Seewald 2013).
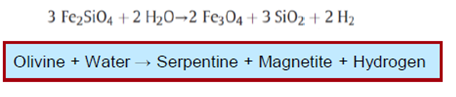
The mantle lithosphere could also support serpentinisation (Brovarone et al. 2017). Low temperature (100 C) H2 production is associated with a spinel reactant and a cubic crystal structure. The spinel can either be as impurities in the initial reactant or the serpentinisation product (Mayhew et al. 2013).
Hydrogen yield from serpentinisation is directly linked with the fraction of reduced iron in the reactant mineral (e.g. olivine or pyroxene with variable Fe/Mg ratios) and the proportion converted to Fe3þ (McCollom and Seewald 2013; Murray et al. 2020).
In felsic rocks (e.g. granites), biotite can also hydrate to generate H2 (Table 1). Nevertheless, high redox potentials, low temperatures and the addition of CO2 can increase H2 yield from granites (Murray et al. 2020).
Radiolysis of water in the geosphere is another significant mechanism of generating natural H2 that is amenable to modelling and resource estimation (Sherwood Lollar et al. 2014; Warr et al. 2019; Boreham et al. 2021. Zgonnik (2020) considers volcanic and hydrothermal vents to be the most dominant H2 source, having the highest estimated natural H2 production rates.
A hydridic source from the lower mantle and core are theorised to be the ultimate largest ‘H’ reservoir (Zgonnik 2020, where H2 can either continually migrate upwards or be released with episodic injection into the upper mantle/crust where the metal hydrides can undergo metasomatism and water hydrolysis.
Biogenic sources
Biogenic sources of natural hydrogen include both thermo-genic and microbial origins.
In the former, H2 is a by-product of diagenesis and thermal maturation, while in the latter, H2 producers and H2 consumers generally work in consortia (Boone et al. 1989; Noar et al. 2015; Gregory et al. 2019) and provide an important H2 sink.
During diagenesis, the polymerisation of hydrocarbon C–C bonds results in the formation of kerogen and minimal release of H2.
Thermogenic liberation of H2 occurs during subsequent catagenic scission of hydrocarbon C–C bonds during the generation of gas and oil, coal seam gas (Gregory et al. 2019, and references therein) and petroleum cracking (Briere et al. 2017). During metagenesis, the progressive aromatisation of the residual kerogen also leads to production predominantly of CH4 but also some H2 (Tissot and Welte 1984; Hunt 1996).
Thermolysis of alkanes and CH4 at 200 C and 500 C, respectively (Tissot and Welte 1984) also result in the release of H2
NATURAL HYDROGEN MIGRATION AND REACTIVITY
Once produced, H2 can react with oxidized elements – mineralized or dissolved in geological fluids – or diffuse toward the surface and escape into the oceans or the atmosphere. This hydrogen can then be either a source of energy for bacterial developments, or as a reagent for abiotic hydrocarbons synthesis, but rarely, if ever, as a carbon-free energy resource. Its high mobility and reactivity at high T, as well as at low T in the presence of bacteria, are considered to prevent its accumulation in the geological media.
This view was recently challenged by two major discoveries of geological environments where H2 is trapped in deep sedimentary formations overlying intra-cratonic crystalline basements.
- Widespread H2 enrichment in water-saturated clay-rich rocks at 20°C surrounding the Cigar-Lake uranium deposits, Athabasca, Canada.
- Thermal desorption measurements reveal that H2 is enriched up to 500 ppm (i.e. 0.25 mol.kg-1 of rock) in these water-saturated rocks having a very low total organic content (<0.5 wt%). Such H2 uptake is comparable and even exceeds adsorbed methane capacities reported elsewhere for pure clay minerals or shales. Up to 17% of H2 produced by water radiolysis over the 1.4 Ga- lifetime of the Cigar Lake uranium ore deposit, accounting for about 500 tones, have been trapped in the surrounding clay alteration haloes. As a result, sorption processes on layered silicates must not be overlooked as they may exert an important control on the fate and mobility of H2 in the crust.
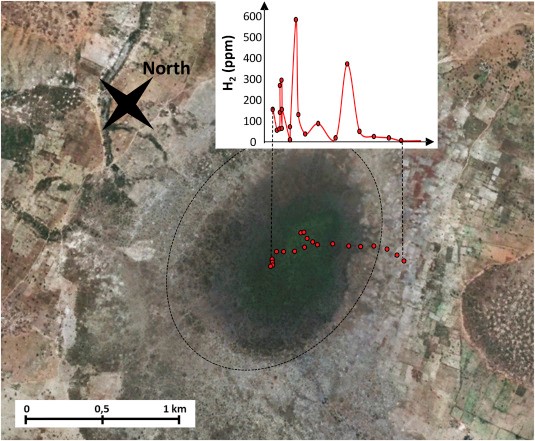
- Large accumulation of H2 in the Taoudeni Basin, Bourakebougou field, Mali.
- Recent exploratory wells in Mali, close to the village of Bourakebougou, confirm the presence of an extensive H2 field featuring at least five stacked reservoir intervals containing significant H2 content (up to 98 vol.% of gas) and covering an estimated area higher than 8 km in diameter. The relatively pure H2 reservoirs are associated with traces of methane, nitrogen and helium. The geological stratigraphic accumulation of H2 is linked to the presence of multi overlaid doleritic sills and aquifers that seem to prevent upward gas migration and leakage.
New experimental data and reactive transport models are needed to investigate H2 transport and storage in deep geological environments. Fundamental data such as H2 solubility, vapor-liquid partitioning, or mineral adsorption under geologically relevant conditions are still lacking. The mechanism for H2 trapping may rely on a combination of both H2 adsorption properties and low solubility acting in concert as an impermeable barrier. Any attempt at modeling H2 behavior in deep geological media will remain misleading in the absence of quantitative data on these two key processes.
EXPLORING FOR NATURAL HYDROGEN
Following the discoveries of oil and gas seeps, the “tipping point” for the petroleum industry was the well drilled by Colonel Drake. The current state of exploration for native hydrogen resembles the beginnings of oil exploration.
Geologists should now adopt the tripartite concept of “source rock, reservoir, and trap” in the exploration for native hydrogen and abiotic gases. Some important parts of the native hydrogen system are already known.
Following a discovery pathway analogous to that of the petroleum industry, the first method of exploration may be to find H2 seeps in areas where source rocks are known. Seeps of native hydrogen and abiotic gases have already been localized in numerous places in subaerial settings.
Three main types of source rocks have already been identified:
- ultrabasic rocks;
- ironrich cratons;
- uraniumrich rocks.
For the first two sources, the production of H2 is linked to the oxidation of Fe(II) by H2O. For the third source, the production of H2 is attributable to the radiolysis of H2O by natural radioactivity. The transformation of H2 into abiotic CH4 can occur under some circumstances through the Sabatier or Fischer–Tropsch reactions (Reeves and Fiebig 2020 this issue). This means that detection of abiotic methane may be a useful indicator for the presence of native hydrogen.
A practical application of the understanding of H2 behavior in the continental crust would be to develop a specifically design exploration guide. Currently, apart from targeting ophiolitic massifs or circular structures in flat sedimentary terrain overlaying cratonic basements, there is no extended exploration guide, nor any dedicated exploration well (apart the recently drilled exploratory wells in Mali).
Knowledge of hydrogen migration and accumulation in the crust is too limited to seriously consider an extensive exploration campaign. This is not surprising since 99% of the deep drilling programs are dedicated to oil and gas exploration/production in geological contexts that are not particularly relevant for hydrogen targeting. Here, we describe what could be a preliminary exploration guide based on a global ‘source-transport-accumulation’ understanding of H2-concentrating process and combining methods used for ore targeting (so-called strategic, tactic and punctual targeting).
- one of the key points related to potential source areas is the presence of gravity and magnetic anomalies that are typically characteristic of iron-rich rocks. These rock formations with strong anomalies, such as ultramafic rocks, or peralkaline granites, can be found at the outcrops but also are observed a few thousand meters below the continental surface. These formations often correspond to ophiolitic sutures or to peridotite massifs sandwiched during orogenic phases. The presence of Archean greenstone belts (e.g. Canadian and Fennoscandian Shields) containing ultramafic rocks may also represent excellent H2-producing zones either via serpentinization, or water radiolysis. There are also intra-cratonic zones (e.g. Russia, North Carolina in the USA, or Mali) that may display significant H2 fluxes, with no clear link with ultramafic rocks. Hydrothermal alteration of other Fe(II)-bearing minerals such as those listed above (amphibole, mica, chlorite) may be envisaged in these latter cases.
- the structural/tectonic context and the presence of faults deeply rooted in the basements capable of draining a potential deep and scattered source will certainly play a very important role. A structural, gravimetric and seismic survey will be relevant to target potentially active deep faults able to drain hydrogen produced at depth where the P-T conditions are optimal.
- if there are storage areas (i.e. a reservoir) of H2 at depth, it will also be necessary to consider the nature of the different lithologies (e.g. mineralogy, porosity, total organic content) and develop prospection methods similar to those implemented for ore targeting. Surface seepages may be either in connection with the source rock or with an intermediate leaking reservoir. Thus, mapping H2 concentration anomaly in soils and outcropping rock formations at different scales will be extremely instructive when superimposed to geological, seismic, and gravimetric data. Many unknown seepages are waiting for explorers to reveal their potential.
Considering the possibility to explore/detect deep reservoir of natural hydrogen in soils or aquifers close to Earth’s surface appear to be severely limited for three reasons:
- the extremely small size of the H2 molecule allows it to diffuse very rapidly through nearsurface environments into the atmosphere
- the high reactivity of H2 with many oxidants (such as Feoxides, Mnoxides, and nitrates) can rapidly destroy H2 in oxidized nearsurface environments
- numerous microorganisms are avid consumers of H2 as a source of energy to support their metabolisms (Ménez 2020 this issue), and these organisms may deplete H2 in shallow environments. On this latter point, a rich literature exists that shows the link between the development of life and the availability of H2 at Earth’s surface (Martin et al. 2008; Russell et al. 2010). All these adverse factors suggest that the most successful exploration efforts are likely to involve drilling to a depth where these nearsurface processes will be excluded
HYDROGEN SYSTEM VS. PETROLEUM SYSTEM
The petroleum system concepts as well as all the tools that allow us to quantify the fluid circulation within the subsurface have been shaped after years of researches and data acquisition. For the hydrogen system, the set of parameters that must be met for a deposit to exist, will have similarities but also differences.
Source, migration pathway, reservoirs, and seals remain mandatory. The various sources of H2 are becoming better known and the rate of the generation appears to be fast. If, as it is subscript in Mali, and as it is clearly the case in all the smokers and volcanic areas, H2 is continuously generated by the water/rock interaction it will be present in the gas, or dissolved gas as long as water is available.
The size of the reservoirs is not an issue, as one may consider a replenishment of the reservoir through human times as for geothermal reservoirs. This represents a main difference with the petroleum systems.
The migration pathways, hence the potential distance between the source rock and the accumulation, remain the least well-known part of the system.

